Whether you’re sitting down at breakfast with a glass of cold orange juice, having a bowl of hot soup for lunch, or enjoying a hot fudge sundae for dessert after dinner, you’re consuming products that most likely are made possible by compressed air. You might think, “Hey, it’s just air.” Maybe it is just air, but there may be substances in that air — and your dessert — that aren’t on the menu.
All-too-common shortcomings
Our company has performed compressed air system audits throughout the United States in dairies, frozen food plants, canning facilities, and dry foods packaging plants. In a typical food processing plant, air compressors are located in the facility’s engine room. There, we frequently find 55-gal drums of lubricant available for use in the compressors. This is not food grade, but synthetic compressor lubricant. The synthetic oil does not necessarily get into the product because filters are in use that remove the oil contamination from the air would be required with any type of oil-flooded or oil lubricated compressors.
As we look over the air filtration in the engine room, Figure 1, we find that the filters are the same type as those found in many other industries — not those designed for the food and beverage processing industry. In addition, it is not unusual for filters to be three months overdue for service or replacement. Moving out onto the production floor, we hope to find adequate filtration used on the actual process machinery. Unfortunately, examining much of the equipment reveals that the point-of-use filtration often is inadequate. Even more disturbing, the compressed air is making incidental — or even direct — contact with the food products or their containers.
Why do we, as compressed air system auditors, notice a few drums of synthetic compressor lubricant? We see synthetic oil all of the time, but we shouldn’t see it in the food processing industry if there is any contact, either direct, or indirect, with the food or beverage product or the containers for holding that product. However, we frequently encounter synthetic compressor oil in food and beverage processing plants all over the United States. In many cases, we find facilities using compressed air containing unacceptable levels of synthetic lubricant to perform tasks such as aerating ice cream, manufacturing beverage containers, and drying the inside of bottles and jars, prior to filling.
Unfortunately, the scenario at the beginning of this article is all too common. We often see the same conditions in plant after plant. Why do they occur, especially since there are regulations concerning compressed air use in the food and beverage industry? How can food and beverage companies correct the problems, when, and if, they become aware of them?
To find answers to these questions, we must take a close look at the food and beverage processing industry’s concerns, needs, and to some extent, its motivations. We also must examine Food and Drug Administration (FDA) regulations, as well as other standards organizations guidelines, concerning compressed air in the industry.
FDA regulations for compressed air
The FDA established clear parameters for compressed air use in Title 21 — Food and Drugs, Chapter 1, Part 110, Section 110.40. Paragraph g. Page 212 reads, “Compressed air or other gases mechanically introduced into food or used to clean food-contact surfaces or equipment shall be treated in such a way that food is not contaminated with unlawful indirect food additives.” Title 21 is updated annually in early summer, but more demanding guidelines have been developed by organizations such as The Dairy Committee and The U. S. Public Health Service.
The International Association of Milk, Food, and Environmental Sanitarians, United States Public Health Service, and The Dairy Industry Committee formulated 3-A Accepted Practices for Supplying Air Under Pressure in Contact with Milk, Milk Products and Product Contact Surfaces, Number 604-04.3-A-604-04 addresses compressed air in seven sections and 29 sub-sections that cover everything from the supply of compressed air, piping, and filtration, to special requirements for handling the air at the point of use.
Contamination — invisible and ignored
Oil contamination in compressed air used in the food and beverage industry occurs largely due to lack of awareness and limited knowledge concerning existing regulations and standards. Most plant managers and engineers at food and beverage plants are unaware of the potential for compressor oil contamination in their facilities because compressed air is, essentially, invisible. This out-of-sight, out-of-mind philosophy is as prevalent throughout the food processing industry as it is among most others. Most people tend to think of compressed air the same way that they think of the air they breathe, not realizing or understanding the process by which compressed air is produced.
Even though many maintenance managers, plant managers, and plant engineers realize that they spend a significant amount of money on compressor lubricants every year, they may never consider the end result of improper application, management, and use of those lubricants in regard to the compressed air supplied to their processes and their products. Too many plants have not updated their processes, integrated new technologies, or kept up-to-date on the latest regulations and standards regarding compressed air supply, distribution, and use. This is the primary causing of compressor oil contaminating the food being processed.
The source: oil-flooded compressors
Many compressed air systems, especially those outside the food processing industry, are supplied by oil-flooded rotary screw type compressors. The oil in these compressors provides a seal when the air is compressed between the rotors and helps cool the rotors and bearings. The rotary screw air end (the rotor pair and bearing assembly that turns inside the housing) discharges air containing a substantial amount of oil.
This air and oil mixture is separated by a filter (sometimes called a de-mister) prior to leaving the compressor. However, even the best rotary-screw compressor, with the most effective and reliable oil separator system, will still pass as much as 4 ppm of compressor oil downstream. This small amount may seem insignificant, but when a compressor is generating 1000 cfm or more of flow, that small amount of oil adds up very quickly.
We audited a midwest dairy that uses a popular synthetic compressor lubricant in all of their oil-flooded rotary- screw compressors. The compressors in this particular facility have an average oil carry-over of 3 to 5 ppm. During our point-of-use analysis, we identified several processes using compressed air where both incidental and direct contact with oil occurred routinely.
Compressed air was used for blow-molding quart and half-gallon milk and juice containers — an example of incidental contact. It was also used to aerate ice cream during packaging (direct contact), and the packaging process for small fruit juice containers supplied to public school systems (both incidental and direct contact). In most areas where compressed air was vented regularly or leaked from the system, discoloration from the compressed air lubricant was easily visible on the contact surfaces, Figure 2.
One might assume that people at this plant were unconcerned with the problem. However, we’ve found that many plants are concerned; they are just not aware of any problem. In a food processing facility we audited for energy savings (as opposed to air quality), we mentioned in passing that they used synthetic compressor lubricant even though processes in their facility brought the compressed air into both direct and incidental contact with products. After a single comment concerning the compressed air quality, and their air’s contact with the product, they immediately changed their system over to food-grade compressor lubricant. In this case, the plant personnel rushed to find an immediate solution, without waiting for our report and recommendations. This demonstrated their willingness and commitment to correcting a problem once they became aware of it.
Compatible compressor lubricants
Food-grade compressor lubricants are one method of addressing an oil contamination problem. These compressor lubricants are available in two varieties:
* full (or direct) contact lubricants, which can be ingested without harm, and
* incidental (or indirect) contact lubricant, which can be ingested in very small amounts without harm. These lubricants have a chemical additive package and are not intended to come into direct contact with the food or beverage product. They tend to be more durable than full contact lubricants and offer a longer oil life cycle than their direct contact counterparts. However, they are not as durable as semi-synthetic or full-synthetic compressor oils.
The advantages of using food-grade lubricants include meeting FDA regulations and U. S. Public Health Service guidelines, as well as reducing the risks of severely contaminating the product. Using food-grade lubricants also enables a plant to use oil-flooded rotary compressors, or lubricated reciprocating compressors. Both cost less initially and require less maintenance than oil-free compressors. The disadvantages of using food-grade lubricants include more frequent compressor oil replacement, higher lubricant cost, lower temperature resistance, and, in some areas, limited availability.
Although large oil-free reciprocating compressors seem to be disappearing from the modern industrial environment, many older machines are still in use today and can be found throughout industry. Today, most oil-free reciprocating compressors are rated less than 30 hp, with some packaged, multi-unit systems rated to 60 hp.
The most common modern designs of oil-free compressors are the rotary screw and the centrifugal varieties. These machines are available in a broad horsepower range from several manufacturers.
The advantages of oil-free compressors for the food and beverage industry are fairly obvious. The risk of compressed air contamination inherent with the use of these machines is almost non-existent. This alone may convince many food and beverage processors that oil-free compressors are their total solution. However, the need for adequate compressed air filtration certainly is not eliminated simply by installing an oil-free compressor. In-line compressed air filters are still needed to ensure that only clean, dry, contaminate-free air makes contact with the product or product container.
The disadvantages to oil free compressors are that they usually carry a higher price tag, and, in many cases, have higher annual maintenance costs than their lubricated counterparts. Some oil-free compressors also have a higher rate of energy consumption, and, therefore may cost more to operate. Because nearly 60% to 70% of a compressor’s total cost over its life span is the cost of electricity, operating cost should not be ignored.
Clean air, no matter what
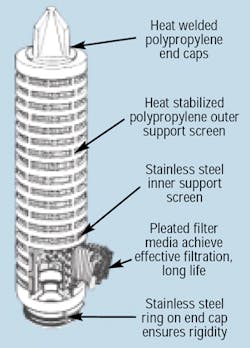
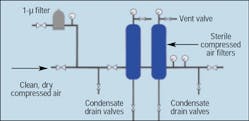
Whether a food processing plant uses lubricated or oil free compressors, proper filtration is still required to ensure that clean air is supplied to the process equipment. Specialized filters — such as activated carbon filters or PTFE-impregnated depth or membrane surface filters — are also required as part of the in-line filtration package. PTFE filters use non-shedding, hydrophobic filter elements to eliminate bacterial contamination in the compressed air stream. These filters, Figure 3, must be cleaned with steam to ensure their continued effectiveness. PTFE filters meet FDA requirements of CFR Title 21 if clean, dry compressed air is supplied to them. Therefore, pre-filtration for these filters is essential in meeting FDA requirements, Figure 4.
It is essential that the compressed air be clean prior to reaching the PTFE filters. But clean, dry air is beneficial for most applications, not just food and beverage processing plants. Clean, dry compressed air can maximize the life and reliability of cylinders, valves, and seals, leading to lower maintenance costs and higher productivity through reduced downtime.
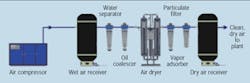
An ideal compressor, filter, and dryer arrangement for providing clean dry air is shown in Figure 5. Unfortunately, pneumatic systems designed and installed with this or comparable arrangements are rare in the most industrial facilities, including the food and beverage industry.
FDA vs. USDA supervision
In every food and beverage processing facility we have visited, we have found at least one USDA representative on site. Each facility also had a testing laboratory. The USDA regularly tests for bacterial contamination of the product, taking random samples of the packaged food or beverage. Not only are detailed records of the test results maintained on file, but the actual item sampled is also stored on site. However, the USDA may not identify chemical contamination of the product unless that contaminant has contributed to bacterial infestation.
Our experience concerning the FDA has been very different. Although the FDA has clearly defined regulations regarding compressed air standards, we have not, in the last two years, seen a single FDA representative in any of the food or beverage facilities we have audited. We cannot claim that they do not visit the plants we have audited — they very well may — but we have never actually encountered an inspector while performing our work. We know that the FDA monitors pharmaceutical manufacturers very closely in regard to compressed air standards. However, our observations seem to suggest that the food and beverage industry seldom comes under close scrutiny by the FDA.
Causes of non-compliance
Once plant personnel become aware that a potential problem exists, they are eager to take action in order to correct the problem. Therefore, a plant audit at a food or beverage processing plant generally consists of:
• informing the client of potential problems and their causes
• suggesting practical solutions to solve the problems
• offering to educate personnel in the areas that will improve operations, and
• providing energy cost savings analyses.
However, compressed air system auditors cannot act as regulatory agents or government inspectors. Their primary commitment is to the client, not the various regulatory or standards organizations. Therefore, clients’ confidentiality takes precedence over any non-compliance issue encountered during the course of an audit.
Although most personnel at food and beverage processing plants try to correct oil contamination situations when they become aware that problems exist, a small percentage (about 5 to 7% from our experience) choose to ignore such problems. Several reasons exist why situations go unresolved, but cost is the primary one. Correcting a non-compliant or potentially non-compliant compressed air system can be expensive. However, in most cases, corrections are not prohibitively expensive. If a plant has operated in violation of FDA regulations for 10 or 15 years without having been caught, there may be little impetus to spend any money to correct a violation that goes unpenalized. The philosophy behind this apathy is simple — if it was not been a problem before, it is not a problem now. Also, a plant may be operating at only a slight profit margin, at a loss, or may be available for acquisition. In these situations, management may view investing capital for a problem that has existed for a long time as out of the question.
Consequences of non-compliance
If the FDA identified a product contamination problem in a food and beverage processing plant, fines would almost surely be levied. If management was aware of problems but chose not to correct them, more drastic action would be taken — such as closing the facility until it became CFR Title 21 compliant.
A facility may also face significant civil penalties if a contaminated product injures or impairs someone. In today’s heavily litigious environment, this is a serious concern that every company should consider as a potential consequence.
What to do
Every facility manager in the food or beverage processing industry should take the time to evaluate his or her compressed air system for potential contamination hazards. Plant personnel should examine their system to determine:
• whether or not their compressed air contains compressor lubricant
• if so, the kind of lubricant in use
• whether or not the compressed air comes into contact with the product or product containers, and
• what kind of filtration is in use.
If a company’s staff feels that it cannot evaluate its compressed air system effectively, then it should consider hiring a skilled, professional compressed air systems consulting company to perform the evaluation. In addition to improving the compressed air quality and helping to bring the plant into FDA compliance, a skilled consultant may also identify energy savings opportunities, offer system performance improvements, improve process equipment up-time, and reduce process equipment maintenance costs.
Most importantly, evaluating the air system can increase awareness of its existence, which overcomes the out-of-sight, out-of-mind attitudes. After all, it is what’s for dinner.
Richard M. Evonitz is director of audit services, and Joseph J. Augustine is auditor, Armstrong Compressed Air Services, Spartanburg, S.C. For more information, visit www.armstronginternational.com.