Download this article in .PDF format
One of the greatest attributes of hydraulics as a method of power transmission is great stiffness, which gives a hydraulic system instant, accurate response. Therefore, one of our chief concerns is to make certain that there is no elastic, power absorbing component in a hydraulic system.
Problems resulting from air
Air in the system has the following major effects:
Spongy control — Because fluids are considered to be basically incompressible, we expect great stiffness in a hydraulic system. That is, the positioning of an actuator should be immediate (rapid response) and precise. The larger the amount of free or entrained air, the spongier (softer, less stiff) the system.
Loss of horsepower — When an air pocket is present in an actuator, it is alternately compressed and relaxed as the actuator is cycled. Since the air pocket must first be compressed before the fluid can cause the actuator to move, power is consumed. Upon relaxation, the air pocket expands and rives fluid out. The stored power, therefore, is expended in driving fluid back into the reservoir and not in moving the actuator.
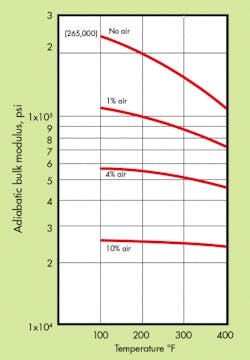
Loss of bulk modulus — Free or entrained air in the hydraulic system reduces substantially the effective bulk modulus of the system. That is, an air-oil mixture appears to increase the compressibility of the fluid, making the system spongy.
Test data seems to indicate that dissolved air has no effect on bulk modulus, providing the air is in solution. These facts, at first, appear paradoxical. However, if one visualizes a container filled to the brim with marbles (which represent the oil molecules) it is possible to pour in a fluid (representing air) around them, or remove the fluid with no change in volume. The weight of the container changes but not the volume.
Loss of system fluid — One of the most serious conditions that can occur in a hydraulic system is the loss of reservoir fluid. The fluid level must be kept high enough to insure enough fluid for the pump intake, otherwise cavitation begins.
A drop in the reservoir level can occur if a large quantity of air, not initially flushed from the lines, makes its way back tot eh reservoir. The reservoir then depletes itself of enough fluid to fill the original air cavity. If the reservoir volume is small and the air cavity is large, the reservoir would become empty or near empty. The discovery of oil depletion may or may not be made in time to protect the pump and prevent aeration of the lines, depending upon reservoir design.
Foaming — Foaming normally occurs in the reservoir because of liquid impinging on the fluid surface, entraining air bubbles. Foaming also results from dissolved air being released because of an increase in fluid temperature. Foaming affects system performance because fluid entering the pump is no longer “solid” but an air-oil mixture, which causes cavitation and also results in a spongy system.
Temperature Effects — A temperature rise in a system containing air affects the bulk of modulus of the air-oil mixture. The bulk modulus, which may be much lower than, desired because of free and entrained air, new drops further. (See graph) An increase in temperature also tends to liberate more dissolved air. This effect is usually evidenced by the increased amount of foaming in the reservoir.
Erosion and cavitation — Cavitation is the formation of a cavity, as a partial vacuum in a fluid or as a gas-filled space in a liquid. One need only look at the gears or pistons of a hydraulic pump, which has been starved of fluid. The result is usually severe surface eroding or pitting of even the hardest materials. The actual mechanics of this erosion are not too well understood. There are theories about “vapor bubble implosion” and “accelerated oxidation”. Regardless of the physics that cause the cavitation, it is something to avoid. To prevent it, a “solid” system is necessary.
Extensive studies of the actual mechanics of erosion are being conducted in several major laboratories. Preliminary data seems to indicate that the presence of dissolved air in system fluid accelerates erosion.
Free air is that trapped in a system, but not totally in contact with a fluid. It is neither entrained nor dissolved; it is entrapped. An example of free air is an air pocket in a system; it can be removed by bleeding.
Entrained air is that suspended in a fluid and normally exists the form of small bubbles. Filters or screens that have a low bubble point can remove entrained air
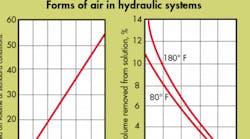
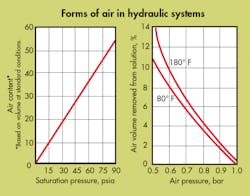
Dissolved air is that in solution in a fluid. Because it is neither free nor entrained air, it does not behave according to Boyle’s law. It does, however obey Henry’s law: the weight of gas dissolved in a liquid is proportional to the pressure of the gas. It can be removed by two methods: subjecting the fluid to a reduced pressure and/or raising the fluid temperature. Its presence or absence does not affect fluid volume.
Dissolved air
Even if all the air bubbles and pockets were removed from a hydraulic system and the system totally enclosed, after a short run-in period, air bubbles would begin to reappear. The source of these bubbles is the working fluid itself because all fluids (except fully dearaeted ones) contain dissolved air. It is this dissolved air that, under certain conditions, comes out of solution and plagues the system.
Adsorption rate
Adsorption, rather than absorption, better describes the process by which bubbles under pressure in a hydraulic fluid are dissolved into the fluid. Adsorb means adhesion of extremely thin films of gases to surfaces with which the gases are in contact. Absorb means to soak up.
The adsorption rate of bubbles (entrained air) in a system is determined by their size. It is true that a fluid holds more dissolved air as pressure increases (Henry’s law). However, the size of the bubble determines at what pressure it will dissolve. Bubbles, with diameters of 0.020 to 0.030 inch, will dissolve at approximately 100 psi. Larger bubbles, however, will not dissolve until the pressure is proportionately greater. This behavior is true even when the amount of previously dissolved air in the fluid is almost nil. We may say, therefore, that the rate of adsorption is a function of the pressure and an inverse function of the bubble diameter. Note that the bubble will reappear when the pressure is lowered.
Origin of dissolved air
Dissolved air will come out of solution when the fluid is exposed to a vacuum. A vacuum can occur in a hydraulic system across orifices; inside un-supercharged pumps; and inside double-acting actuators when driven by an external load, at a rate speed greater than the rate at which fluid can fill the opposite end.
Dissolved air, once out of solution, can be partly readsorbed in a moving stream when the local static pressure again exceeds 14.7 psia. When a stream containing bubbles is suddenly stopped, however, the bubbles will begin to migrate upwards and coalesce in the nearest high point. Repressurization may or may not drive the new, larger bubble back into solution.
Techniques of air removal and measurement
There is free air in a system before it is filled. Conventional fill and flush techniques will remove the major portion of it. However, some pockets will remain, depending n component and circuit design. Without a very low-vacuum fill procedure, it is next to impossible to remove them. When filling under vacuum, the fill fluid should be deaerated; otherwise it will release air when it comes in contact with the vacuum. Deareted fluid could adsorb some of the air in these pockets.
Where you deal with entrained air in an open-reservoir-system, a low bubble-point filter or mesh will help screen out the entrained air bubbles. To remove them entirely, the fluid should be processed through a deaerator as described above. Also, reservoir return lines should be placed well below the fluid level to minimize circulation and vortices.
Dissolved air is the hardest of the three types of air to remove y conventional means. The bulk of it can only be removed in the presence of a vacuum.
Creating a vacuum in a reservoir to remove dissolved air only damages the pump. (One of the primary purposes of a pressurized reservoir is to prevent pump cavitation, which occurs when a vacuum is created at the pump inlet.) Since the amount of dissolved air removed is proportional to the degree of vacuum, the best technique is again to use a deaerator, which will remove all forms of air in a circulating system.
Note that even when a deaerator of large capacity is used continuously in a system, the deaerator can bring an open system’s dissolved air content down only so far before the deaeration rate equals the rate of readsorption of air taking place in the open reservoir.
This article originally appeared in the October 1967 issue of Hydraulics & Pnematics. It is republished here for its continuing technical value.