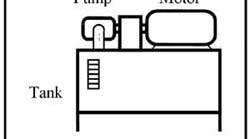
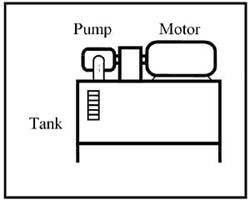
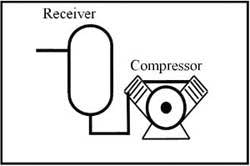
Any media (liquid or gas) that flows naturally or can be forced to flow could be used to transmit energy in a fluid power system. The earliest fluid used was water hence the name hydraulics was applied to systems using liquids. In modern terminology, hydraulics implies a circuit using mineral oil. Figure 1-1 shows a basic power unit for a hydraulic system. (Note that water is making something of a comeback in the late '90s; and some fluid power systems today even operate on seawater.) The other common fluid in fluid power circuits is compressed air. As indicated in Figure 1-2, atmospheric air -- compressed 7 to 10 times -- is readily available and flows easily through pipes, tubes, or hoses to transmit energy to do work. Other gasses, such as nitrogen or argon, could be used but they are expensive to produce and process.
Of the three main methods of transmitting energy mechanical, electrical, and fluid fluid power is least understood by industry in general. In most plants there are few persons with direct responsibility for fluid power circuit design or maintenance. Often, general mechanics maintain fluid power circuits that originally were designed by a fluid-power-distributor salesperson. In most facilities, the responsibility for fluid power systems is part of the mechanical engineers' job description. The problem is that mechanical engineers normally receive little if any fluid power training at college, so they are ill equipped to carry out this duty. With a modest amount of fluid power training and more than enough work to handle, the engineer often depends on a fluid power distributor's expertise. To get an order, the distributor salesperson is happy to design the circuit and often assists in installation and startup. This arrangement works reasonably well, but as other technologies advance, fluid power is being turned down on many machine functions. There is always a tendency to use the equipment most understood by those involved.
Fluid power cylinders and motors are compact and have high energy potential. They fit in small spaces and do not clutter the machine. These devices can be stalled for extended time periods, are instantly reversible, have infinitely variable speed, and often replace mechanical linkages at a much lower cost. With good circuit design, the power source, valves, and actuators will run with little maintenance for extended times. The main disadvantages are lack of understanding of the equipment and poor circuit design, which can result in overheating and leaks. Overheating occurs when the machine uses less energy than the power unit provides. (Overheating usually is easy to design out of a circuit.) Controlling leaks is a matter of using straight-thread O-ring fittings to make tubing connections or hose and SAE flange fittings with larger pipe sizes. Designing the circuit for minimal shock and cool operation also reduces leaks.
A general rule to use in choosing between hydraulics or pneumatics for cylinders is: if the specified force requires an air cylinder bore of 4 or 5 in. or larger, choose hydraulics. Most pneumatic circuits are under 3 hp because the efficiency of air compression is low. A system that requires 10 hp for hydraulics would use approximately 30 to 50 air-compressor horsepower. Air circuits are less expensive to build because a separate prime mover is not required, but operating costs are much higher and can quickly offset low component expenses. Situations where a 20-in. bore air cylinder could be economical would be if it cycled only a few times a day or was used to hold tension and never cycled. Both air and hydraulic circuits are capable of operating in hazardous areas when used with air logic controls or explosion-proof electric controls. With certain precautions, cylinders and motors of both types can operate in high-humidity atmospheres . . . or even under water.
When using fluid power around food or medical supplies, it is best to pipe the air exhausts outside the clean area and to use a vegetable-based fluid for hydraulic circuits.
Some applications need the rigidity of liquids so it might seem necessary to use hydraulics in these cases even with low power needs. For these systems, use a combination of air for the power source and oil as the working fluid to cut cost and still have lunge-free control with options for accurate stopping and holding as well. Air-oil tank systems, tandem cylinder systems, cylinders with integral controls, and intensifiers are a few of the available components.
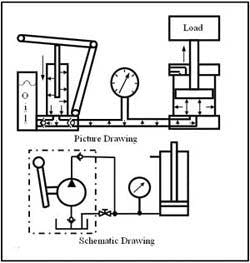
The reason fluids can transmit energy when contained is best stated by a man from the 17th century named Blaise Pascal. Pascal's Law is one of the basic laws of fluid power. This law says: Pressure in a confined body of fluid acts equally in all directions and at right angles to the containing surfaces. Another way of saying this is: If I poke a hole in a pressurized container or line, I will get PSO. PSO stands for pressure squirting out and puncturing a pressurized liquid line will get you wet. Figure 1-3 shows how this law works in a cylinder application. Oil from a pump flows into a cylinder that is lifting a load. The resistance of the load causes pressure to build inside the cylinder until the load starts moving. While the load is in motion, pressure in the entire circuit stays nearly constant. The pressurized oil is trying to get out of the pump, pipe, and cylinder, but these mechanisms are strong enough to contain the fluid. When pressure against the piston area becomes high enough to overcome the load resistance, the oil forces the load to move upward. Understanding Pascal's Law makes it easy to see how all hydraulic and pneumatic circuits function.
Notice two important things in this example. First, the pump did not make pressure; it only produced flow. Pumps never make pressure. They only give flow. Resistance to pump flow causes pressure. This is one of the basic principles of fluid power that is of prime importance to troubleshooting hydraulic circuits. Suppose a machine with the pump running shows almost 0 psi on its pressure gauge. Does this mean the pump is bad? Without a flow meter at the pump outlet, mechanics might change the pump, because many of them think pumps make pressure. The problem with this circuit could simply be an open valve that allows all pump flow to go directly to tank. Because the pump outlet flow sees no resistance, a pressure gauge shows little or no pressure. With a flow meter installed, it would be obvious that the pump was all right and other causes such as an open path to tank must be found and corrected.
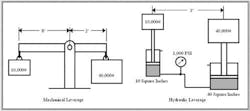
Another area that shows the effect of Pascal's law is a comparison of hydraulic and mechanical leverage. Figure 1-4 shows how both of these systems work. In either case, a large force is offset by a much smaller force due to the difference in lever-arm length or piston area.
Notice that hydraulic leverage is not restricted to a certain distance, height, or physical location like mechanical leverage is. This is a decided advantage for many mechanisms because most designs using fluid power take less space and are not restricted by position considerations. A cylinder, rotary actuator, or fluid motor with almost limitless force or torque can directly push or rotate the machine member. These actions only require flow lines to and from the actuator and feedback devices to indicate position. The main advantage of linkage actuation is precision positioning and the ability to control without feedback.
At first look, it may appear that mechanical or hydraulic leverage is capable of saving energy. For example: 40,000 lb is held in place by 10,000 lb in Figure 1-4. However, notice that the ratio of the lever arms and the piston areas is 4:1. This means by adding extra force say to the 10,000-lb side, it lowers and the 40,000-lb side rises. When the 10,000-lb weight moves down a distance of 10 in., the 40,000-lb weight only moves up 2.5 in.
Work is the measure of a force traversing through a distance. (Work = Force X Distance.). Work usually is expressed in foot-pounds and, as the formula states, it is the product of force in pounds times distance in feet. When a cylinder lifts a 20,000-lb load a distance of 10 ft, the cylinder performs 200,000 ft-lb of work. This action could happen in three seconds, three minutes, or three hours without changing the amount of work.
When work is done in a certain time, it is called power. {Power = (Force X Distance) / Time.} A common measure of power is horsepower - a term taken from early days when most persons could relate to a horse's strength. This allowed the average person to evaluate to new means of power, such as the steam engine. Power is the rate of doing work. One horsepower is defined as the weight in pounds (force) a horse could lift one foot (distance) in one second (time). For the average horse this turned out to be 550 lbs. one foot in one second. Changing the time to 60 seconds (one minute), it is normally stated as 33,000 ft-lb per minute.
No consideration for compressibility is necessary in most hydraulic circuits because oil can only be compressed a very small amount. Normally, liquids are considered to be incompressible, but almost all hydraulic systems have some air trapped in them. The air bubbles are so small even persons with good eyesight cannot see them, but these bubbles allow for compressibility of approximately 0.5% per 1000 psi. Applications where this small amount of compressibility does have an adverse effect include: single-stroke air-oil intensifiers; systems that operate at very high cycle rates; servo systems that maintain close-tolerance positioning or pressures; and circuits that contain large volumes of fluid. In this book, when presenting circuits where compressibility is a factor, it will be pointed out along with ways to reduce or allow for it.
Another situation that makes it appear there is more compressibility than stated previously is if pipes, hoses, and cylinder tubes expand when pressurized. This requires more fluid volume to build pressure and perform the desired work. In addition, when cylinders push against a load, the machine members resisting this force may stretch, again making it necessary for more fluid to enter the cylinder before the cycle can finish.
As anyone knows, gasses are very compressible. Some applications use this feature. In most fluid power circuits, compressibility is not advantageous; in many, it is a disadvantage. This means it is best to eliminate any trapped air in a hydraulic circuit to allow faster cycle times and to make the system more rigid.
Boyle's Law
Boyle's Law for gasses states: It is the principle that, for relatively low pressures, the absolute pressure of an ideal gas kept at constant temperature varies inversely with the volume of the gas. In down-home language this means if a ten cubic foot volume of atmospheric air is squeezed into a one cubic foot container, pressure increases ten times. (10 X 14.7 psia = 147 psia.) Notice that pressure is stated as psia.
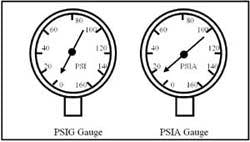
Normally, pressure gauges read in psi (with no additional letter). Commonly called gauge pressure, psi disregards the earth's atmospheric pressure of 14.7 psia, because it has no effect either negative or positive on a fluid power circuit. The a on the end of psia stands for absolute, and would be shown on a gauge with a pointer that never goes to zero unless it is measuring vacuum. Another type of gauge that shows both negative and positive pressures would have a pointer with an inches-of-mercury (in. Hg) scale below zero and a psig scale above zero. Both of these gauges could read pressure or vacuum. (They are always found in a refrigeration repairperson's tool kit. Refrigeration units have both vacuum and pressure in different sections of the system at the same time.) Figure 1-5 pictures a typical psig gauge and one type of psia gauge.
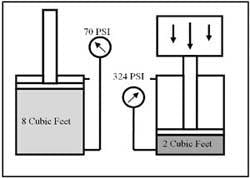
In the example above, when ten cubic feet of air was squeezed into a one cubic-foot space, both pressures were given in psia. To see what gauge pressure (psig) would be, subtract one atmosphere from the 147-psia reading. (147 psia 14.7 psia = 132.3 psig.) To calculate the amount of compression of air in a system, always use absolute pressure, or psia, not psig. For example: the cylinder in Figure 1-6 contains eight cubic feet of air at 70 psig. To what will pressure increase when an external force pushes the piston back until the space behind the piston is two cubic foot? It is obvious the pressure will rise four times. At first it might look easy to take 70 psig X 4 = 280 psig, but this answer is wrong. For the correct answer, gauge pressure must be changed to absolute pressure. In this case by adding one atmosphere to the 70-psig reading. (70 psig + 14.7 psia = 84.7 psia.) Now multiply the 84.7-psia pressure by 4 to see what the absolute pressure is when the cylinder stops at one cubic foot volume. (84.7 X 4 = 338.8 psia.) Finally, to return to gauge pressure, subtract one atmosphere from the absolute pressure. (338.8 psia 14.7 psia = 324.1 psig.) Notice that the correct pressure is 44.1 psig higher than when gauge pressure is the multiplier.
Temperature was not considered in both preceding cases, but notice that the law says kept at constant temperature. Compressing a gas always increases its temperature because the heat in the larger volume is now packed into a smaller space. The next law says that increasing temperature increases pressure if the gas cannot expand. This means the pressures given are measured after the gas temperature returns to what it was originally.
Gauges today read in psi and bar. Bar is a metric or SI unit for pressure and is equal to approximately the barometer reading or one atmosphere. One atmosphere is actually 14.696 psi but the SI unit for bar is 14.5 psi.
Charles' Law
Heating a gas or liquid causes it to expand. Continuing to heat a liquid will result in it changing to the gaseous state and perhaps spontaneous combustion. If the gas or liquid cannot expand because it is confined, pressure in the contained area increases. This is stated in Charles' Law as: The volume of a fixed mass of gas varies directly with absolute temperature, provided the pressure remains constant. Because fluid power systems have some areas in which fluid is trapped, it is possible that heating this confined fluid could result in part damage or an explosion. If a circuit must operate in a hot atmosphere, provide over pressure protection such as a relief valve or a heat- or pressure-sensitive rupture device. Never heat or weld on any fluid power components without proper preparation of the unit.
Static head pressure
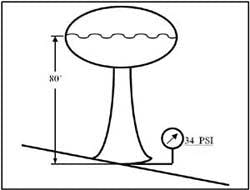
The weight of a fluid in a container exerts pressure on the containing vessel's sides and bottom. This is called static head pressure. It is caused by earth's gravitational pull. A good example of head pressure is a community water system. Figure 1-7 shows a water tower with a topmost water level of 80 feet. A cubic inch of water weighs 0.0361 pounds. Therefore a one square-inch column of water will exert a force of 0.0361 psi for every inch of elevation. This works out to .433 psi per foot of elevation. For the water tower in Figure 1-7, the pressure at the base would be: 80 ft X 0.433 psi/ft = 34.6 psi. This pressure is always available, even when no pumps are running. Of course, if the water level drops, static head pressure also will drop.
The specific gravity of hydraulic oil is approximately 0.9, so multiplying water's 0.433 psi per foot by 0.9 shows oil exerts 0.39 psi per foot of elevation. Usually this fraction is rounded to 0.4 for simplicity. If the water tower were filled to 80 ft with oil, it would exert a pressure of 32 psi at ground level. Other fluids would develop a higher or lower static pressure according to their specific gravities.
This pressure is only realized at ground level at the tower. Outlets at other levels would be higher or lower according to their distance below the fluid surface.
Tanks seen on most water towers simply store volume. Pressure does not drop rapidly or require frequent pump starts to maintain the fluid level. The size or shape of the tank does not affect pressure at the base. Pressure at the base of a straight 80-ft pipe would be the same, but useful volume before pressure drop would change drastically. Always remember: it is not the physical size of a body of fluid that determines pressure but how deep it is.
Head pressure can have an adverse effect on a hydraulic system because many pumps are installed above the fluid level. This means the pump must first create enough vacuum to raise the fluid and then create even higher vacuum to accelerate and move it. Therefore there is a limit to how far a pump can be located above the oil level. Most pumps specify a maximum suction pressure of 3 psi. At 4- to 5-psi suction pressure, pumps start to cavitate . . . causing internal damage. At 6- to 7-psi vacuum, cavitation damage is severe and noise levels increase noticeably. (The effects of cavitation are covered fully in Chapter 8, Fluid power pumps and accessory items.) Axial- or in-line-piston pumps are especially vulnerable to high inlet vacuum damage and should be set up below the fluid level to produce a positive head pressure.
Many modern hydraulic systems place the pump next to the reservoir so the fluid level is always above the pump inlet. With this type of installation the pump always has oil at startup and has a positive head pressure at its inlet. A better arrangement puts the tank above the pump to take advantage of even greater head pressure. Everything possible should be done to keep pressure drop low in the pump inlet line because the highest possible pressure drop allowable is one atmosphere (14.7 psi at sea level).
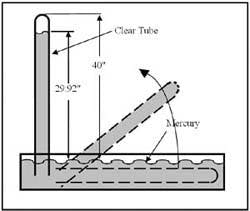
The earth's atmosphere the air we breathe exerts a force of 14.7 psi at sea level on an average day. This pressure covers the whole earth's surface, but at elevations higher than sea level, it is reduced by approximately 0.5 psi per 1000 feet. This pressure of earth's atmosphere is the source of the power of vacuum. The highest possible vacuum reading at any location is the weight of the air above it at that time. A reading of maximum vacuum available is given during the local weather forecast as the barometer reading. Divide the barometer reading by two to get the approximate atmospheric pressure in psi. This force could be directly measured if it were possible to isolate a one square-inch column of air one atmosphere tall at a sea level location. Because this is not possible, the method used to measure vacuum is demonstrated in Figure 1-8.
Submerge a clear tube with one closed end in a container of mercury and allow it to fill completely. (The tube must be more than 30-in. long for this example to work when mercury is the liquid.) After the mercury displaces all the air in the tube, carefully raise the tube's closed end, keeping the open end submerged so the mercury can't run out and be replaced by air. When the tube is positioned vertically, the liquid mercury level will lower to give the atmospheric pressure reading in inches of mercury (29.92-in. Hg at sea level). The mercury level will fluctuate from this point as high and low-pressure weather systems move past. If the tube had been 100-in. tall, the mercury level would still have dropped to whatever the atmospheric pressure was at its location. The reason the mercury does not all flow out is that atmospheric pressure holds it in.
This barometer could have been built using another liquid but the tube would have to be longer because most other liquids have a much lower specific gravity than mercury's 13.546. Water, with a specific gravity of 1.0, would require a closed-end tube at least 33.8 ft long, while oil, with a specific gravity of approximately 0.9, would have to be even longer.
Vacuum pumps can be similar in design to air compressors. There are reciprocating-piston, diaphragm, rotary-screw, and lobed-rotor designs. (See air compressor types in Chapter 8, Fluid power pumps and accessory items.) Imagine hooking the inlet of an air compressor to a receiver tank and leaving the outlet open to atmosphere. As the pump runs, it evacuates air from the receiver and causes a negative pressure in it.
Vacuum pumps are an added expense and normally are only found in facilities that use a constant supply of negative pressure to operate machines or make products.
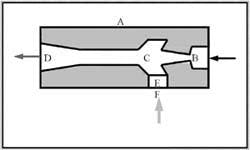
Vacuum generators that use plant compressed air as a power source are also available. These components have no moving parts but use plant air flowing through a venturi to produce a small supply of negative pressure. Figure 1-9 shows a simplified cutaway view of a venturi-type vacuum generator. The device consists of body A with compressed-air inlet B that passes air flow through venturi nozzle C. The air exhausts at a higher velocity to atmosphere through orifice D. As air at increasing velocity flows past opening E near the venturi nozzle, it creates a negative pressure and draws in atmospheric air through port F. Port F can connect to any external device that needs a vacuum source. A vacuum gauge at port F shows negative pressure when compressed air is supplied to port B.
Vacuum generators are inexpensive, but can be costly to operate. For every 4 cfm of air supply required to power them, they use approximately one compressor horsepower. For this reason, venturi-type vacuum generators usually are installed with a control valve to turn them on only when needed.
Vacuum is limited to one atmosphere maximum at any location, and standard vacuum pumps only reach about 85% (approximately 12 psi) of this on average. As a result, vacuum is not powerful enough to do much work unless it acts on a large area.
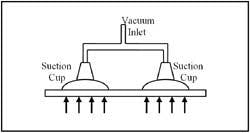
Many industrial vacuum applications have to do with handling parts. Large-area suction cups can lift a large heavy part with ease, as illustrated in Figure 1-10. When the lift rises, negative pressure (vacuum) inside the suction cups causes atmospheric pressure on the opposite side of the part to push it up.
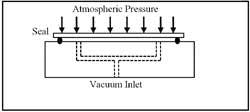
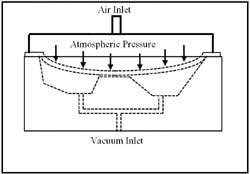
Industries such as glass and wood manufacturing use vacuum to hold work pieces during machining or other operations, as shown in Figure 1-11. The pieces are held firmly in place as the negative pressure under them causes atmospheric pressure to push against them. A resilient seal laid in a groove in the fixture keeps atmospheric air from entering the cavity beneath the part. This groove can be cut to match the contour of the part. In machining operations, the seals can isolate interior cutouts, allowing them to be removed while firmly holding the rest of the piece.
Heated plastic sheet can be vacuum-formed to make some products at a much lower cost than other types of plastic forming, as suggested in Figure 1-12. Forming heated plastic sheet in a cavity or over a shape is quick and positive. When atmospheric pressure tries to fill the negative-pressure area under the softened sheet, the sheet is forced into the desired shape. Large parts such as pickup-truck bed liners are formed by this method.